JAK Inhibitor Risk Assessment Tool
Assess Your Risk
This tool helps you understand your individual risk of infections and blood clots while taking JAK inhibitors based on your medical history and lifestyle factors.
When you’re managing a chronic autoimmune condition like rheumatoid arthritis or psoriatic arthritis, finding a treatment that actually works can feel like a breakthrough. JAK inhibitors - drugs like tofacitinib, upadacitinib, and baricitinib - deliver real relief for many people. They block specific signals in the immune system that cause inflammation, often working faster and more effectively than older biologics. But for every benefit, there’s a hidden risk. Two of the most serious side effects you need to know about are infections and blood clots.
Why JAK Inhibitors Increase Infection Risk
JAK inhibitors don’t just quiet down the bad immune signals - they also dampen the ones your body uses to fight off germs. This isn’t a minor side effect. It’s built into how the drugs work. Studies tracking over 120,000 patients show that serious infections occur in about 3% to 5% of people taking these medications each year. That’s roughly double the rate seen in those on traditional DMARDs or even some biologics.The most common serious infection is herpes zoster - also known as shingles. Even if you’ve been vaccinated, the risk doesn’t disappear. One patient on Reddit shared that they developed shingles within three months of starting tofacitinib, despite getting the Shingrix shot. They ended up hospitalized for five days with nerve pain and skin lesions. This isn’t rare. In fact, shingles makes up nearly 15% of all infection reports linked to JAK inhibitors.
Other dangerous infections include tuberculosis (TB), fungal infections like histoplasmosis, and bacterial pneumonia. Some patients develop sepsis from minor cuts or urinary tract infections that spiral out of control. The reason? JAK inhibitors interfere with interferon signaling, which is critical for controlling viruses and intracellular bacteria. Your body’s early warning system gets muted.
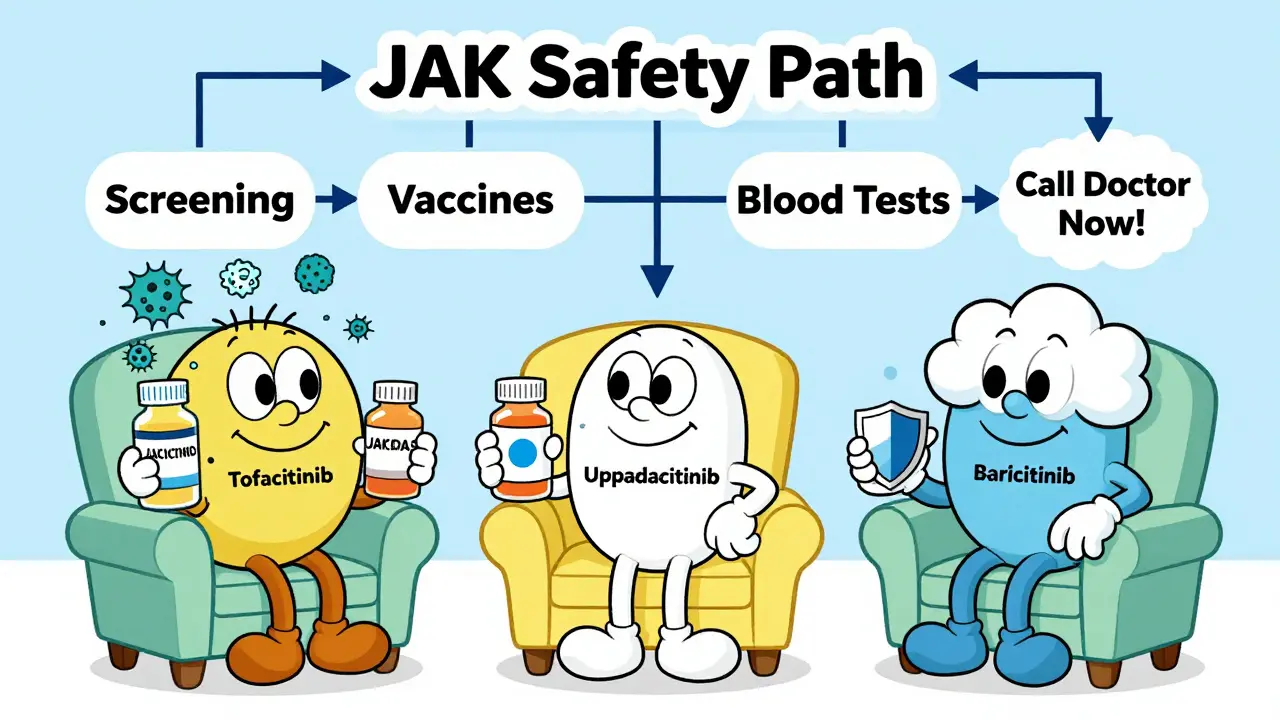
Before starting treatment, you need to be screened for latent TB with a skin test or blood test. You also need to be up to date on vaccines - but not just any vaccines. Live vaccines like MMR, varicella, and nasal flu spray are absolutely off-limits once you’re on a JAK inhibitor. You must get them at least four weeks before starting. If you’re not sure about your vaccination history, get tested. It’s not optional.
Thrombosis: The Silent Threat
While infections are more common, blood clots are more dangerous. Venous thromboembolism (VTE) - which includes deep vein thrombosis (DVT) and pulmonary embolism (PE) - is the second major red flag. The FDA added a black box warning for this in 2021 after a large study showed JAK inhibitors more than doubled the risk of clots compared to TNF inhibitors.Here’s what the data says: Patients on tofacitinib had a 73% higher risk of pulmonary embolism and a 54% higher risk of death from any cause. Even newer drugs like upadacitinib carry this risk, though slightly lower. The mechanism? JAK2 inhibition reduces thrombopoietin signaling, which affects platelet production and clotting balance. It’s not just about being sedentary or flying long distances - although those are triggers. The drug itself changes your blood’s clotting landscape.
High-risk patients include those over 65, current or former smokers, people with a history of blood clots, obesity (BMI over 30), or those on estrogen therapy. One patient on upadacitinib described getting a DVT in their calf after a long flight. Their rheumatologist stopped the drug immediately. That’s standard protocol. If you suddenly have swelling, pain, or warmth in one leg - especially if it’s only on one side - don’t wait. Get checked. If you’re short of breath or have chest pain, go to the ER. A pulmonary embolism can kill within hours.
Not All JAK Inhibitors Are the Same
It’s easy to think of JAK inhibitors as one group. They’re not. Their safety profiles vary based on which JAK enzymes they block.- Tofacitinib (Xeljanz) blocks JAK1 and JAK3 most strongly, with some JAK2 activity. It has the highest reported rates of clots and infections.
- Upadacitinib (Rinvoq) is highly selective for JAK1. Early data shows a lower VTE signal - about 0.2 events per 100 patient-years versus 0.9 for tofacitinib in low-risk patients.
- Baricitinib (Olumiant) inhibits JAK1 and JAK2. Its clot risk sits between the two.
- Filgotinib (Jyseleca) is JAK1-selective with almost no JAK2 effect. It’s not available in the U.S., but in Europe, it’s often chosen for patients with higher clot risk.
This matters. If you’re over 65 and have a history of clots, your doctor should consider a JAK1-selective agent instead of a pan-JAK inhibitor. The difference isn’t just theoretical - it’s life-saving.

What You Must Do Before and During Treatment
Starting a JAK inhibitor isn’t like picking up a new painkiller. It requires a structured safety plan.- Get screened. TB test, hepatitis B and C screening, and a full blood count. Check your lipid levels - JAK inhibitors raise cholesterol within weeks.
- Update vaccines. Pneumococcal, flu, hepatitis B, and Shingrix. No live vaccines after starting.
- Assess your risk. Do you smoke? Are you over 65? Have you had a clot before? Do you have heart disease or diabetes? If yes to any, your doctor must document why this drug is still the best option.
- Monitor closely. Blood tests every 4 to 8 weeks for the first few months. Watch for low white blood cells, low platelets, or rising cholesterol. If your LDL jumps 20%, your doctor may start a statin.
- Know the warning signs. Fever, chills, cough, or unexplained fatigue? Could be infection. Swelling in one leg? Could be a clot. Don’t wait. Call your doctor the same day.
Some clinics now use digital risk-assessment tools that auto-flag high-risk patients before the prescription is even written. In Australia, the TGA requires prescribers to document these checks. If your doctor doesn’t ask about your clot history or vaccination status, push back. This isn’t just caution - it’s standard of care.
What Happens If You Have a Side Effect?
If you develop a serious infection - like pneumonia, sepsis, or active TB - the drug must be stopped immediately. You won’t restart it until the infection is fully cleared. Sometimes, that means months off the medication.If you get a blood clot, you’ll be switched to anticoagulants like warfarin or a direct oral anticoagulant (DOAC). The JAK inhibitor is permanently discontinued. No exceptions. Even if it was working perfectly, the risk isn’t worth it.
Some patients worry that stopping the drug means going back to joint pain or skin flare-ups. That’s true. But there are alternatives. Biologics like TNF inhibitors, IL-17 blockers, or even newer TYK2 inhibitors are still options. The goal isn’t to avoid treatment - it’s to avoid deadly complications.
Real Patients, Real Choices
Patient satisfaction with JAK inhibitors is mixed. On Drugs.com, the average rating is 6.2 out of 10. About 42% of negative reviews mention infections. 28% mention blood clots. But 82% of those who didn’t have complications say the drugs changed their lives.One 71-year-old woman with severe rheumatoid arthritis stopped tofacitinib after a pulmonary embolism. She switched to a TNF inhibitor and now walks without pain. She says, "I’d rather have a little less relief than risk dying from a clot." Another patient, 54, started upadacitinib after failing three biologics. No infections, no clots. Her psoriasis cleared. She says, "It’s the best thing that’s happened to me in 10 years. I just stay on top of my blood tests."
The message isn’t "avoid JAK inhibitors." It’s "know your risks and manage them."
The Bigger Picture
After the 2021 safety warnings, JAK inhibitors went from being a top choice for rheumatoid arthritis to a second-line option in most guidelines. Prescribing rates dropped from 35% to 28% of new biologic starts. TNF inhibitors are back in favor. But JAK inhibitors still have a place - especially for patients who can’t tolerate injections or don’t respond to biologics.Regulators in the U.S., Europe, and Australia all agree: these drugs aren’t for everyone. They’re for carefully selected patients who understand the risks and are monitored closely. The data shows that when used right - with screening, vaccination, and vigilant monitoring - the benefits still outweigh the risks.
But if you skip the checks, ignore the symptoms, or assume "it won’t happen to me," you’re gambling with your life. This isn’t about fear. It’s about awareness.
Can I still get vaccinated while on a JAK inhibitor?
You can get inactivated vaccines like flu, pneumococcal, hepatitis B, and Shingrix while on a JAK inhibitor. But live vaccines - such as MMR, varicella, and the nasal flu spray - are dangerous and must be avoided. Get all necessary live vaccines at least four weeks before starting the drug. Once you’re on it, no live vaccines are safe.
How often do I need blood tests on JAK inhibitors?
You need a complete blood count (CBC) every 4 to 8 weeks for the first 3 to 6 months. After that, if your numbers are stable, you may drop to every 3 months. You also need a lipid panel at 4 weeks and again at 12 weeks to check for rising cholesterol. These aren’t optional - they’re how you catch problems early.
Are newer JAK inhibitors safer than older ones?
Yes, newer agents like upadacitinib and filgotinib are more selective for JAK1 and have less effect on JAK2, which is linked to clotting. Early data shows lower rates of venous thromboembolism compared to tofacitinib. But they’re not risk-free. Long-term data is still being collected. If you’re high-risk for clots, your doctor may prefer one of these newer options.
What should I do if I feel sick while on a JAK inhibitor?
Don’t wait. If you have a fever over 100.4°F, chills, a new cough, unexplained fatigue, or red, swollen skin - call your doctor immediately. These could be signs of infection. For sudden leg swelling, chest pain, or shortness of breath, go to the emergency room. These are signs of a blood clot. Early action saves lives.
Is it safe to take JAK inhibitors if I’ve had cancer before?
The FDA and EMA warn against using JAK inhibitors in patients with a history of cancer, especially if it was recent or aggressive. These drugs may increase the risk of cancer recurrence or new cancers. If you’ve had cancer, your doctor should only consider a JAK inhibitor if no other treatment works - and even then, they’ll weigh the risks very carefully.
Next Steps for Patients
If you’re considering a JAK inhibitor, ask your doctor these questions:- Have I been screened for TB and hepatitis?
- Am I up to date on all non-live vaccines?
- Do I have any risk factors for blood clots?
- Is there a JAK1-selective option that might be safer for me?
- What’s the plan if I get an infection or a clot?
If you’re already on one, make sure you’re getting your blood tests on time. Keep a symptom journal. Note any fevers, rashes, swelling, or unusual fatigue. Bring it to your next appointment. The more you know, the safer you’ll be.
JAK inhibitors aren’t the enemy. But they’re not harmless either. Treat them like a powerful tool - one that needs careful handling. Get the facts. Ask the hard questions. Stay alert. Your health depends on it.


